Last Updated: Mar 19, 2021
Guidelines Used:
- 2020 IDSA COVID-19, Part 1: Treatment and Management of Patients with COVID-19
- 2020 NIH Coronavirus Disease 2019 (COVID-19) Treatment Guidelines
In the last year, COVID-19 has spread worldwide rapidly, increasing incidence of infections and deaths. The Infectious Diseases Society of America (IDSA) and National Institute of Health (NIH) have spearheaded efforts to provide updated evidence-based guidelines to support treatment and management of patients with COVID-19 with researchers all over the world.
Based on a request, here is a summary of coronavirus-19 disease (COVID-19) guidelines and clinical trials **as of March 19, 2021**. Evidence is rapidly changing; please be diligent in viewing the latest evidence-based data.
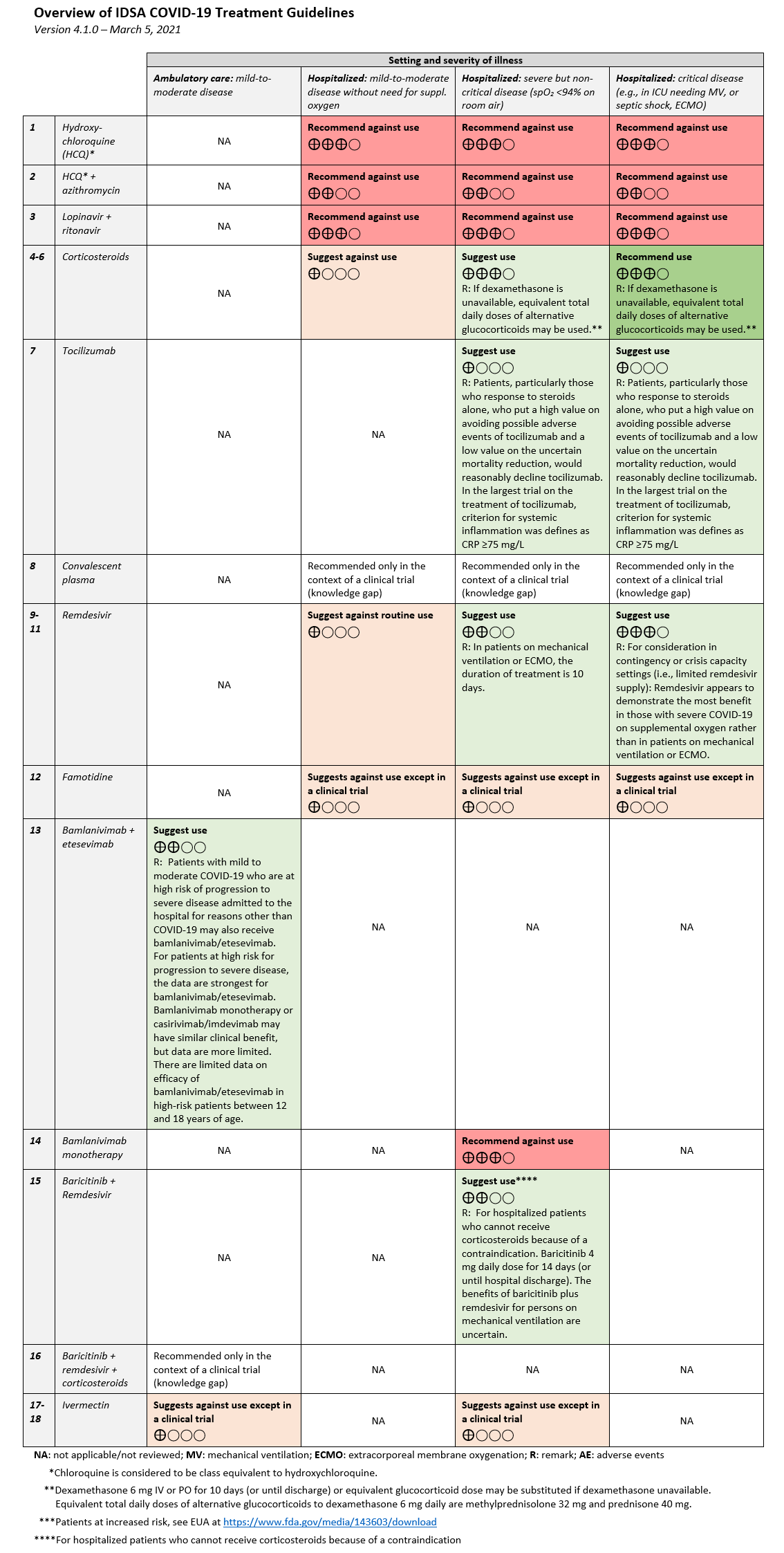
COVID-19 Compiled Clinical Trials with their Therapies (as of March 19, 2021)
| Therapy | MOA | Associated Clinical Trials |
| Hydroxychloroquine | Antimalarial | Barnabas et al, 8 December 2020Mitjà et al. 24 November 2020Self et al, 9 November 2020Rajasingham et al, 17 October 2020RECOVERY Collab group, 8 October 2020Abella et al, 30 September 2020Skipper et al, 16 July 2020RECOVERY, 5 June 2020Boulware et al, 3 June 2020 |
| Hydroxychloroquine + azithromycin | Antimalarial + macrolide antibiotic | Cavalcanti et al, 23 July 2020 |
| Azithromycin | Macrolide antibiotic | PRINCIPLE, 21 March 2021Furtado et al, 4 September 2020 |
| Lopinavir + ritonavir | HIV type 1 aspartate protease inhibitor + booster | RECOVERY, 5 October 2020Horby et al, 29 June 2020 Hung et al, 8 May 2020Cao et al, 7 May 2020 |
| Corticosteroids | Anti-inflammatory steroid | RECOVERY Collab Group, 25 February 2021Tomazini et al, 2 September 2020Horby et al, 17 July 2020 |
| Tocilizumab | Monoclonal antibody that inhibits interleukin (IL-6) | Soin et al, 4 March 2021REMAP-CAP Investigators, 25 February 2021Veiga et al, 20 January 2021Salama et al. 17 December 2020Stone et al, 21 October 2020Hermine et al, 20 October 2020Salvarani et al, 20 October 2020 |
| Tocilizumab + Remdesivir | Monoclonal antibody + antiviral | REMDACTA (Roche), 11 March 2021 |
| Convalescent plasma | Recovered COVID-19 patient’s plasma | Libster et al, 18 February 2021REMAP-CAP trial 12 January 2021Simonovich et al, 24 November 2020Agarwal et al, 22 October 2020Li et al, 3 June 2020 |
| Remdesivir | Broad spectrum antiviral | Solidarity (WHO), 2 December 2020Beigel et al, 8 October 2020Spinner et al, 21 August 2020Goldman et al, 27 May 2020Wang et al, 29 April 2020 |
| Famotidine | Histamine-2 receptor antagonist | Multi-Site Adaptive Trials for COVID-19, ongoing |
| Bamlanivimab + etesevimab | Neutralising IgG1 monoclonal antibody directed against SARS-CoV-2 spike protein | Gottlieb et al, 21 January 2021 |
| Bamlanivimab (LY-CoV555) | *same as above | BLAZE-2, 21 January 2021Chen et al, 21 January 2021ACTIV-3, 26 October 2020 |
| Baricitinib + Remdesivir | Janus-associated tyrosine kinase (JAK) 1 and 2 inhibitor (modulates immune response) | Kalil et al, 11 December 2020 |
| Ivermectin | Anti-parasitic | López-Medina et al, 4 March 2021 |
References
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed March 19, 2021.
- IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Infectious Diseases Society of America. Available at https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#. Accessed March 19, 2021.